Maintaining cryogenic temperatures with pressure control
Cryogens are substances that liquefy at or below 120 K under standard pressure (760 Torr). They are commonly used in applications to keep liquefied substances at extremely cold temperatures, including cryopreservation, rocket propulsion, and medical imaging.
Two of the most well-know cryogens are liquid nitrogen and helium. Liquid nitrogen is commonly used for cold storage and flash freezing in lab settings, and is near the upper end of the cryogen temperature spectrum. Helium is particularly notable for its proximity to absolute zero (0 K). It is typically at 3-4 K, with variations depending on the specific isotope and the ambient pressures.
Challenge: Mitigating loss of cryogenic gases
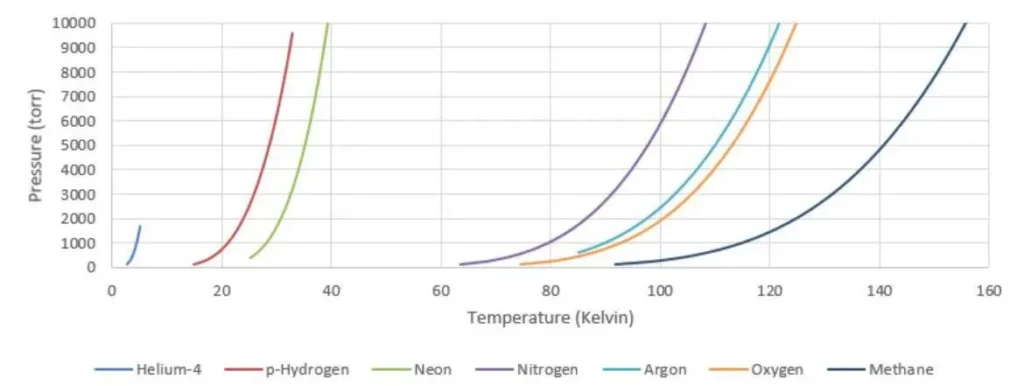
The equilibrium temperature of a cryogen is highly dependent on pressure, as demonstrated in figure 1. Because of this, tight control of absolute pressure enables tight control of absolute temperature.
In cryopreservation applications, researchers must prevent the loss of the cryogen due to evaporation and pressure fluctuations. This is challenging, as atmospheric pressure often fluctuates by as much as ±25 Torr.
Imagine a researcher using a 100 L dewar of liquid helium. A pressure decrease of 25 Torr will result in the vaporization of 0.874 liquid liters, generating 25 standard cubic feet (or ~700 standard liters) of helium gas. This is approximately $25 worth of liquid helium. Over a period of years, the helium vaporization may quickly amount to thousands of dollars in unnecessary loss.
Figure 1: Interdependence of temperature and pressure for common cryogens.
In cryopreservation applications, researchers must prevent the loss of the cryogen due to evaporation and pressure fluctuations. This is challenging, as atmospheric pressure often fluctuates by as much as ±25 Torr.
Imagine a researcher using a 100 L dewar of liquid helium. A pressure decrease of 25 Torr will result in the vaporization of 0.874 liquid liters, generating 25 standard cubic feet (or ~700 standard liters) of helium gas. This is approximately $25 worth of liquid helium. Over a period of years, the helium vaporization may quickly amount to thousands of dollars in unnecessary loss.
In an extreme example, pressure fluctuations in propellant storage tanks at NASA’s John F. Kennedy space center resulted in boiloff of 650 kg of liquid hydrogen each day – estimated in 2001 to cost $625,000 each year!
Solution: Tightly regulating headspace pressure
Pressure meters/controllers therefore play critical roles in cryogenic temperature control, including:
- Monitoring cryogen loss from closed volumes
- Maximizing efficiency of cryogen recovery systems
- Controlling back pressure in cryogenic storage containers
- Controlling temperature of cryogenic experiments via absolute pressure control
Method 1: Maintaining headspace pressure with back pressure control
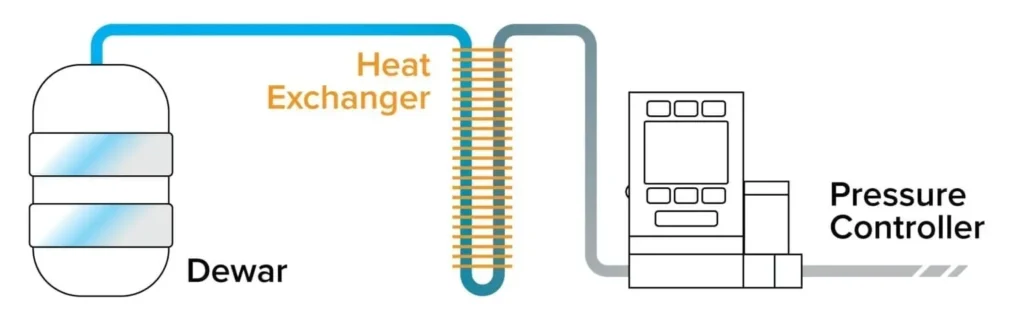
Absolute pressure controllers can be used to maintain a constant absolute pressure in the headspace of the dewar, ensuring minimal cryogen loss. When the helium recovery system is utilized, the exhaust port of a back pressure controller should be connected directly to the recovery manifold, rather than venting to atmosphere as in Figure 2. This is a simple and low-cost method of precisely controlling back pressure of cryogenic systems.
Figure 2: Back pressure controller used to control headspace pressure in a cryogen dewar and minimize helium loss.
With this technique, the only source of positive pressure in the system is the boiloff created by heating the cryogen. Therefore, any attempt to control the absolute temperature of the system using pressure changes is dependent on the time it takes to boil off a certain amount of the cryogen (generally a slow process). Some people use a pressure building coil to speed up the pressure increase and subsequent warming.
Method 2: Active headspace pressure control
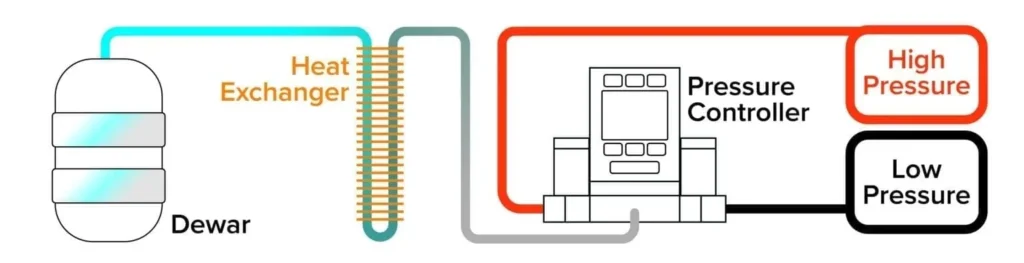
For faster, more flexible control of cryogen pressures and temperatures, it can be better to use an active headspace pressure control scheme. Attaching both a positive pressure source of a warm gas and a vacuum system to a dual valve pressure controller will give you greater speed and better control of the cryogen pressure and temperature.
The configuration shown in Figure 3 allows you to rapidly introduce the warm gas to increase the system pressure. This effect is improved by the increased rate of boiloff as the cryogen shifts its equilibrium conditions. Conversely, the pressure of the system can be lowered by venting to atmosphere or a vacuum. As the pressure decreases, cryogen temperature will decrease as well.
Figure 3: A dual valve pressure controller used to actively control pressure in the headspace of a cryogen dewar
In this way, the cryogen temperature rapidly increases or decreases while you are maintaining precise control. Because the vapor pressure of any liquid is a function of temperature, the temperature of a liquid in thermal equilibrium with its saturated vapor is a function of its absolute pressure.