Converting CO2 to ethanol flow
CO2 is considered the primary fossil fuel emission related to global warming for many different manufacturing and chemical applications. As a result, there is much research to develop more efficient methods to reduce CO2 emissions through means of carbon capture, sequestration, and conversion.
In contrast, ethanol is a well-known inebriant, disinfectant, and energy source. In fact, roughly 10% of modern gasoline fuel blends contain ethanol primarily derived from corn biomass. Due to these reasons, ethanol is inherently valuable, trading for about $1000 per ton with a global market size of $75 billion per year.
In 2020, researchers developed a highly energy efficient, low variable cost, carbon-neutral or carbon-negative, electrochemical process for converting CO2 directly into ethanol. As most of the modern ethanol supply originates from biomass, mass adoption of CO2 conversion as an alternative ethanol production technique will help to lower alcohol and food costs while concurrently reducing CO2’s impact on global warming.
Below we will describe how this conversion works in depth as well as how Alicat’s mass flow controllers can be used to optimize the process.
Electrocatalytic CO2 reduction to ethanol
The CO2 reduction reaction pathway
Electrocatalytic CO2 reduction using different electrocatalysts made from semiconductors with suitable band alignments or compatible metal and semiconductor nanoparticles produces a series of valuable fuels and chemicals under visible light irradiation. By changing the configuration of semiconductors and metals used, different chemicals are produced. Notably, copper catalysts create various types of end products, including a wide range of valuable hydrocarbons and oxygenates.
In some configurations, these reactions occur in an electrolysis cell and in others they occur in an electrolytic medium between an anode and cathode. In some system designs, the CO2 is sparged with deionized water to humidify it before electrolysis, ensuring more even power generation to keep the system more healthy.
When using copper catalysts, by controlling for variables such as electrolyte pH, electrode potential, molecular additives, and electrolyte cation design, the activity and selectivity of the CO2RR pathway can be customized to produce different end products such as ethylene (C2H4), ethanol (C2H5OH), and propanol (C3H7OH).
A 91% FE CO2 to ethanol conversion process
A highly efficient CO2 to ethanol process was developed by Argonne National Laboratory’s Center for Nanoscale Materials and APS. Their process used carbon-supported copper catalysts to achieve a low power, low cost, CO2 to ethanol pathway with an FE of 91% at just −0.7 V potential (versus the reversible hydrogen electrode) with an onset potential as low as −0.4 V (reversible hydrogen electrode).
In this configuration, pure CO2 was added into a 6.8 PH electrochemical cell containing a catalyst-coated graphene sheet made of special single atom carbon-supported copper electrocatalysts acting as the working electrode, an Ag/AgCl reference electrode, and a platinum wire counter electrode. A 0.1 M KHCO3 solution acted as the electrolyte. Constant CO2 flow was maintained throughout the test at 30 standard cubic centimeters per minute (sccm) for 4,000 seconds.
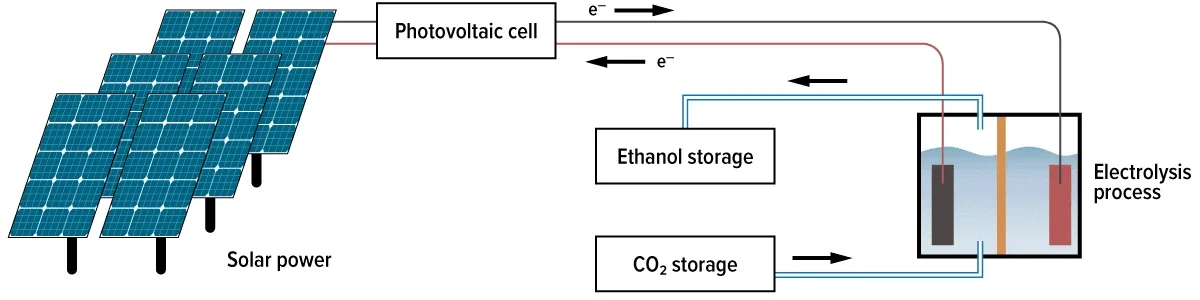
CO2 electrolysis flow control using Alicat devices
Alicat’s MC-Series of mass flow controllers are designed to optimize the flow of CO2 by maintaining repeatable and accurate data and control across a large range of flow rates in addition to constantly monitoring operating pressure and temperature, allowing for more precise electrochemical cell operation and testing conditions.
- Flow accuracy up to ±0.6% of reading or ±0.1% of full scale
- Repeatability up to ± 0.1% of reading + 0.02% of full scale
Alicat’s CODA KC-Series of Coriolis mass flow controllers are compatible for high humidity liquid or gas flow control and can be used universally for all different types of electrolysis systems. Additionally, CODA KC-Series operates at a wide range of high and low pressures, allowing for the same device to be used to control various fluid mixes. CODA flow controllers work well for the low flow rates used in this experiment, with full-scale ranges down to 40 g/h (and up to 100 kg/h).
- Flow accuracy for liquids up to ±0.6% of reading or ±0.2% of full scale and with a repeatability of just ±0.1% of full scale
- Flow accuracy for gases is accurate up to ±1% of reading or ±0.2% of full scale, whichever is greater.
Additional support
Alicat’s flow and pressure controllers can be used in additional electrolysis applications, including direct regulation of other electrolysis systems, test benches for membrane and catalyst verification, fuel cell system modelling, and more.
Alicat’s mass flow and pressure controllers provide operating solutions for various electrolysis applications. Whether you are using electrolysis for ethanol or hydrogen production, or for making other chemicals, we can optimize your procedure.